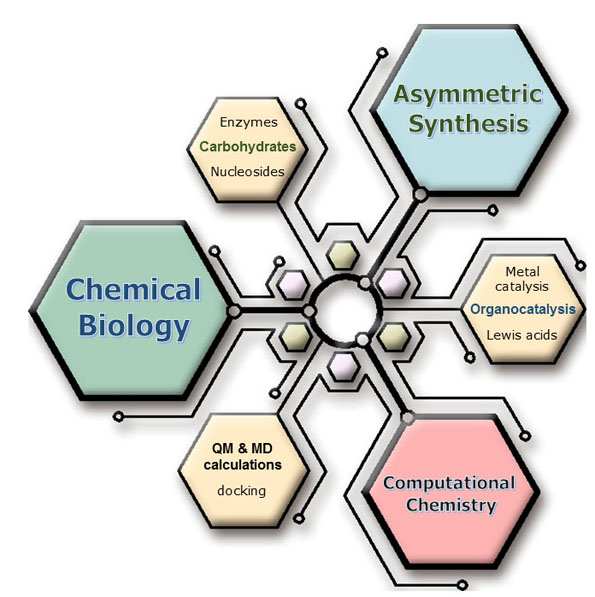
We develop three main research lines in our group, all of them closely related. We based our approaches in novel strategies of Asymmetric Synthesis with a direct application in Chemical Biology. Computational Chemistry gives support to both disciplines.
We use asymmetric catalysis, including both organocatalysis and metal-catalysis, and stereoselectve synthesis for synthesizing enantiomerically pure compounds with biological activities as inhibitors / modulators of key enzymes. In particular we dedicated ourefforts to the preparation of glycomimetics (carbohydrate derivatives and nucleoside analogs). QM (quantum mechanics) and MD (molecular dynamics) calculations are used for elucidating mechanisms and studying protein-ligand interactions.
(click the area for going directly to the research line) |
|
| Research Lines |
|
Highlights |
| |
|
|
Glycosyltransferases (GTs)
|
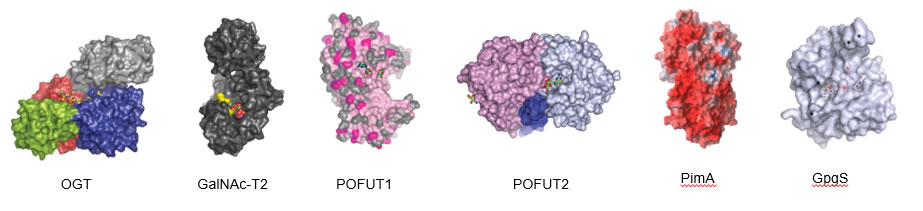
Glycosyltransferases (GTs) constitute a large family of enzymes (E.C. 2.4.x.y) and each family consists of subfamily members that create the same linkage with the same sugar donor but transfer to different sugar acceptors. In this Project we focus our attention in GTs involved in protein glycosylation. These molecules of enormous diversity mediate a wide range of functions from structure and storage to signalling and, in consequence, they are related with important diseases. We focus our attention on a series of target enzymes involved in protein glycosylation which takes place by using U- and G-sugars as Leloir donors. Figure shows several examples of enzymes studied in our group.
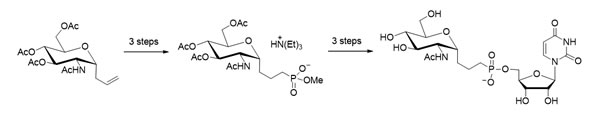
The asymmetric synthesis of glycoconjugates with nucleotide analogs bearing the alpha-phosphate unit has served for claryfing the role of the pyrophosphate moiety.
The interactions of this derivative with the enzyme GalNAC-T2 has been studied and compared with that of uridine, present in the natural substrate in which the glycosyl unit is N-Ac-galactosamine
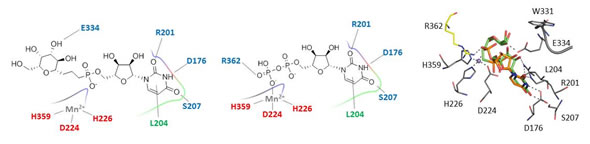
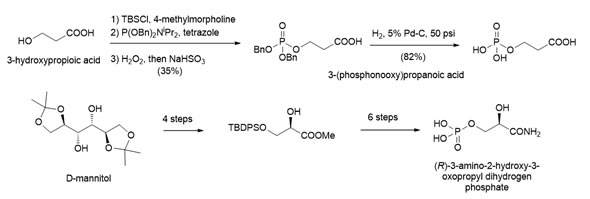
Collaboration with the group of Dr. Guerin allowed trapping a native tertiary complex for the enzyme GpgS by preparing fosforylated derivatives of 3-hydroxypropionic acid and chiral non-racemic 2-hydroxy-propanamide.
To understand the mechanism of the enzymes will be crucial for designing ligands/inhibitors pursued in our research. For that purpose |
Transglycosylases (TGs)
|
In addition, we have identified key residues for enhancing recognition close to the active site that could be used for anchoring additional elements to the basic recognition unit. These preliminary results allowed to us, for the present project, to focus our efforts in optimizing the designed structures towards nm values and, more importantly, extend our studies to AfGel4, essential for the pathogenic Aspergillus fumigatus, responsible of aspergillosis
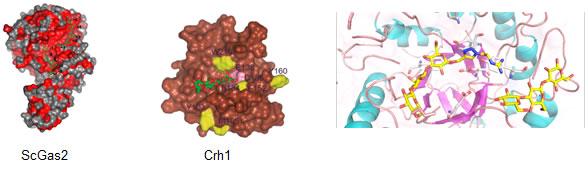
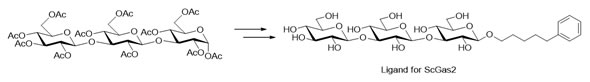
Other target enzymes include Crh1 that use chitin as the donor substrate and chitin, β-1,3 and β-1,6 glucan as acceptor substrates in order to assemble the yeast cell wall. MD and NCI studies with ScGas2 are being used for studying the mode of action and protein-ligand interactions of these enzymes. A suitable ligand for ScGas2 has been prepared from peracetylated laminaritriose.
 |
Design of Glycomimetics
|
We are interested in novel glycomimetics, rationally designed by analyzing carbohydrate-protein interactions, to be evaluated against GTs and TGs processing carbohydrates. We aim to explore the ability of the designed glycomimetics to mediate, modulate and inhibit the target enzymes and to convert the understanding of these processes into useful biotools by applying the recent advances in the field, including modern asymmetric organic synthesis, crystallographic analysis, computational tools, NMR studies, etc. We set up a synthetic methodology directed to prepare β-(1,3)-glucomimetics that have shown some interaction with ScGas2 and gave Kd values in the order of units of µM.
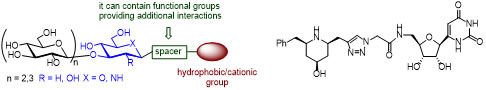
Stereoselective allylation and propargylation reactions of chiral nitrones allowed the preparation of key intermediates for accessing to a series of glycomimetics.
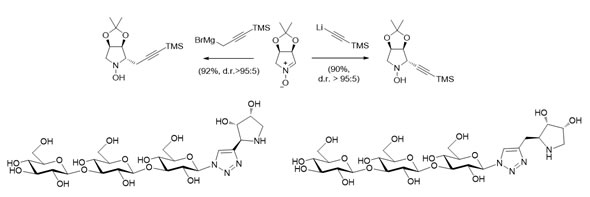 |
QM Calculations
|
The elucidation of reaction mechanisms is carried out by using Gaussian09 licensed software. Several studies have been carried out. Among them the thionation reaction of carbonyl compounds with Lawesson’s reagent (LR) has been computationally studied using DFT methods and topological analyses. the reaction takes place through a two-step mechanism involving (i) a concerted cycloaddition between one monomer and the carbonyl compound to form a four-membered intermediate and (ii) a cycloreversion leading to the thiocarbonyl derivative and a ArPOS monomer.
The oxime-nitrone tautomerism was revisited using high-level DFT calculations. The isomerization was found to be more favorable through a bimolecular process involving two molecules of oxime, a finding that argues against the commonly accepted thermal 1,2-H-shift mechanism. The reaction of arylamidoximes with 1,2-diaza-1,3-dienes to yield the corresponding O-substituted oximes resulted to take place through the corresponding nitrone revealing the dramatically different reactivity predicted for arylamidoximes and unsubstituted oximerationalized in terms of steric hindrance.
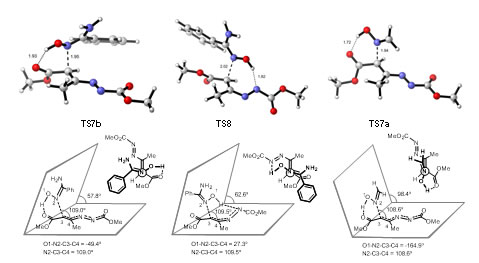
NCI studies reveal non covalent interactions helping to the understanding of driving forces in the chemical reactions. These studies can be also applied to protein´ligand interactions studies. In the reaction of lithium ynolates with nitrones, NCI studies in conjunction with ELF topological studies corroborated that the reaction is, actually, one-step kinetic process but consisting of two sages, the formation of the correspponding bonds being sequential.
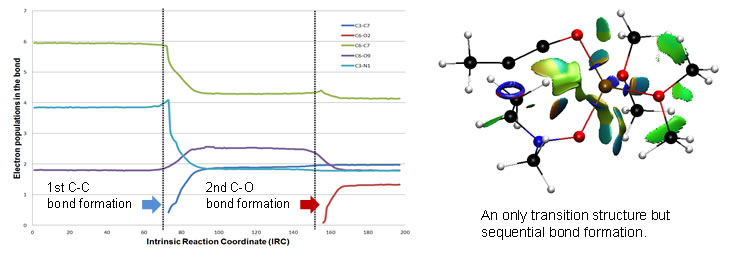
The figure illustrates the electron population in the bonds during the course of the reaction and NCI situation at the transition structure. |
| MD Calculations |
Recent research shows that molecular dynamics simulations are crucial for describing biological mechanisms and that new drug targets and new therapies can be developed based on its understanding. It is well-known that only a small fraction of the known proteins have a known function, and for even fewer their structures are described, necessary information for the rational design of small molecule therapeutics. While the cost and difficulty of determining protein structures remains high, there is ever increasing evidence of the importance of dynamics and disordered states in most biological processes. These states are generally inaccessible to structural studies which in an increasing number, reveal the existence of alternative, transient binding sites on proteins that are formed upon ligand binding. The prediction of such dynamic binding sites is of major interest for the invention of ligands with novel, highly specific biological impact.
We carried out MD calculations with AMBER16 licensed software
to modelize both transglycosylases and glycosyltransferases. The figure illustrates a study carred out with GalNAc-T2 and a suitable ligand in collaboration with the group of Prof. S. Martin-Santamaria at CIB-CSIC (Madrid).
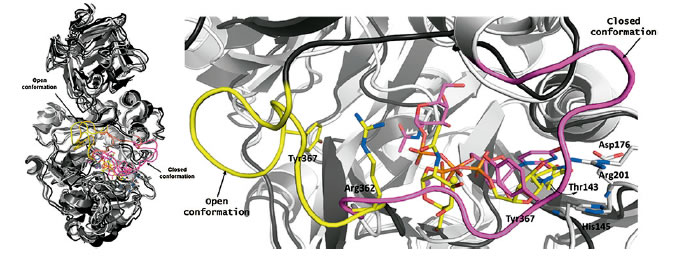
In particular, superposition of five structures of the GalNAc-T2/UDP-GalNAc complex along the tMD simulation of the transition from the open (black, loop in yellow) to the closed (white, loop in magenta) conformation is showed |
| Docking Calculations |
We perform docking studies by using both free software (AutoDock, Gold ) and licensed software (Schrodinger and Discovery Studio ). All complex structuresare retrieved from the PDB or achieved from our X-ray studies carried out in collaboration with structural biological chemists. By using docking studies it was determined that three key residues of monosaccharide are crucial for enhancing recognition close to the active site of transglycosylase ScGas2. They could be used for anchoring additional elements to the basic recognition unit. These results allowed to us to focus our efforts in optimizing the designed structures towards nm values for ScGAs2 and extending our studies to AfGel4, essential for the pathogenic Aspergillus fumigatus, responsible of aspergillosis.
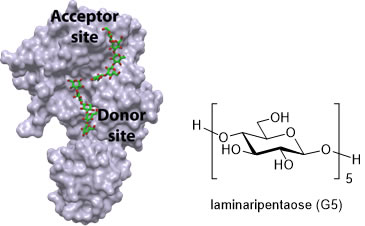 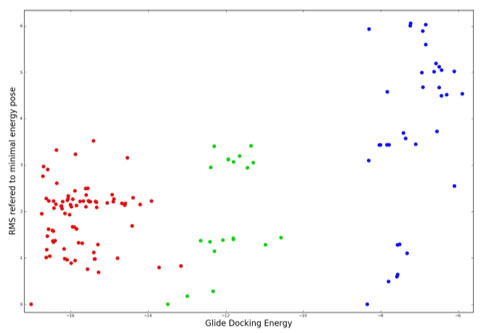
The figure illustrates the enzyme ScGas2 which acts as a model for the correspondgin enzyme Gel4 essential in Aspergillus fumigatus responsible of Aspergillosis and graphical representation of docking energies |
| |
| Metal-Catalysis |
Chiral non-racemic Lewis acids are used in a variety of reactions including nucleophilic additions to nitrones, dipolar cycloadditions, Michael additions, etc. to induce asymmetry. A rhodium-based system in which the formation of the dipolarophile/catalyst complex is favored with respect to nitrone coordination represents a rare example of efficient activation of electron-deficient monofunctionalized alkenes toward the 1,3-dipolar cycloaddition reaction of nitrones.This activation leads to 3,4-isoxazolidines as major regioisomers with enantioselectivities up to 92%.
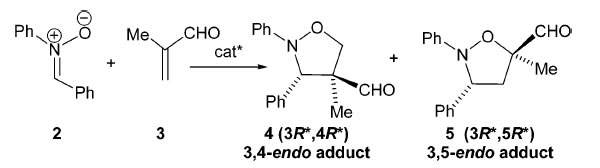
Currently, we are working on developing the asymmetric version of the recently developed Li-catalyzed synthesis of 3-oxazolines from nitrone ylides. |
| Organocatalysis |
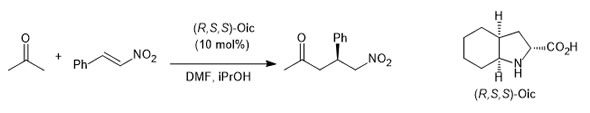
Novel organocatalytic processes including tandem reactions, cycloadditions, nucleophilic additions to C=X systems and Friedel Crafts reactions are studied in our group using well-known modes of activation based on amine catalysis (enamine/iminium) and hydrogen bonding. The Michael addition of acetone to nitroalkenes can be catalyzed by OICs. The reaction takes place with good yields and enantioselectivities.
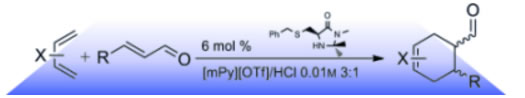
An organocatalytic Diels-Alder reaction has been developed in colaboration with the group of Profs. A. De Nino and L. Maiuolo from the University of Cosenza (Italy).
The mechanism of organocatalytic reactions are also studied computationally in order to determine the mode of action and design novel and more efficient catalysts. As an example, we have studied the mechanism of action of bifunctional catalysts derived from squaramides and pyrrolidines and determined that two different models can apply. The study has been carried out in collaboration with Prof. K. A. Jorgensen (Aarhus University, Denmark) and Prof. J. L. Vicario (University of Basque Country, Spain) |
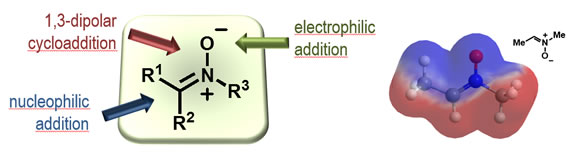
We develop new asymmetric syntheses mainly focused to nitrogen-containing compounds through the development of novel methodologies based on the reactivity of the nitrone functionality as both electrophile in nucleophilic addition reactions and dipole in dipolar cycloadditions towards intermediates of synthetic utility.
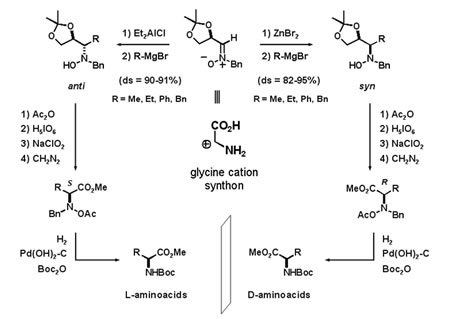
It is possible to carry out stereocontrolled nucleophilic additions by the appropriate use of Lewis acids. This reactivity served to prepare a series of enantiomerically pure organic compounds of biological interest such as aminoacids, carbohydrates and nucleoside analogues.
Aminoacid nucleosides such as the antibiotic Polyoxin J have been prepared by using synthetic methodologies developed in our laboratories.
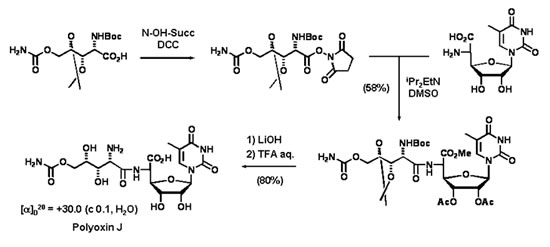
We developed new methodologies using hitherto unknown azomethine ylides derived from nitrones (nitrone ylides). These methodologies arebased on above mentioned asymmetric catalysis. The complementarity and versatility of the different synthetic approaches are exploited for the preparation of compounds with a high functional diversity. Those compounds are further used for synthesizing more complex compounds of biological interest, including highly functionalized pyrrolidines and piperidines.
|
| |